Dietary exposure to silver nitrate compared to two forms of silver nanoparticles in rainbow trout: bioaccumulation potential with minimal physiological effects†
Abstract
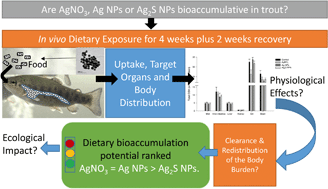
The trophic transfer of silver to fishes in aquatic food chains is a concern, but little is known about the dietary accumulation of pristine (Ag NPs) and modified (Ag2S NPs) forms of silver-containing nanoparticles. The current study aimed to assess the bioaccumulation potential of these materials following dietary exposure to 100 mg kg−1 Ag as either AgNO3, Ag NPs or Ag2S NPs compared to no added Ag controls. The experiment consisted of a 4 week uptake phase, followed by a further 2 weeks on the control diet (total 6 weeks). Fish were sampled for total Ag analysis (weeks 1–4 and 6), plasma ions, biochemistry and histology. The highest Ag concentrations were in the mid intestine, hind intestine, kidney, liver and gallbladder, regardless of the type of silver exposure. Overall, there was significantly more Ag accumulation from AgNO3 and Ag NP exposure compared to the Ag2S NP treatment, indicating a lower bioavailability of the latter. Following the 4 week exposure, the highest Ag concentrations (from AgNO3, Ag NPs and Ag2S NPs, respectively) was in the hind intestine (140, 90, 0.5 μg g−1), liver (120, 130 and 11 μg g−1) and gallbladder (20, 40 and 1 μg g−1). The liver concentration represented around 40% of the body burden of Ag for all Ag treatments. Following the depuration period (week 6), the Ag concentrations in the tissues showed some transient changes. Notably, there was a significant increase in the liver Ag body burden (66, 63 and 99% for AgNO3, Ag NPs and Ag2S NPs, respectively) in the post-exposure phase. An in chemico digestibility assay simulating low pH in the stomach indicated some dissolution of silver, but there were equal orders of Ag release from both the AgNO3 and Ag NP diets, and none from the Ag2S NPs. There were no treatment-dependent differences in cumulative food intake or intestinal morphology, and only minor transient changes in plasma ions, total glutathione and TBARS in the organs. Overall, the dietary bioaccumulation potential of the nano forms of silver was equal to, or less than the metal salt, and with minimal toxicological effects following 4 weeks exposure.


 Please wait while we load your content...
Please wait while we load your content...