Crystalline nickel, cobalt, and manganese antimonates as electrocatalysts for the chlorine evolution reaction†
Abstract
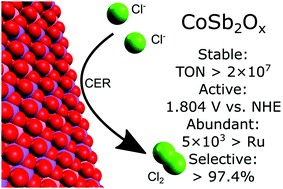
The chlorine-evolution reaction (CER) is a common, commercially valuable electrochemical reaction, and is practiced at industrial scale globally. A precious metal solid solution of RuO2 or IrO2 with TiO2 is the predominant electrocatalyst for the CER. Herein we report that materials comprised only of non-precious metal elements, specifically crystalline transition-metal antimonates (TMAs) such as NiSb2Ox, CoSb2Ox, and MnSb2Ox, are moderately active, stable catalysts for the electrochemical oxidation of chloride to chlorine under conditions relevant to the commercial chlor-alkali process. Specifically, CoSb2Ox exhibited a galvanostatic potential of 1.804 V vs. NHE at 100 mA cm−2 of Cl2(g) production from aqueous pH = 2.0, 4.0 M NaCl after 250 h of operation. Studies of the bulk and surface of the electrocatalyst and the composition of the electrolyte before and after electrolysis indicated minimal changes in the surface structure and intrinsic activity of CoSb2Ox as a result of Cl2(g) evolution under these conditions.


 Please wait while we load your content...
Please wait while we load your content...
