Diastereoselective diazenyl formation: the key for manganese-catalysed alcohol conversion into (E)-alkenes†
Abstract
The proposed reaction mechanism for the unprecedented direct transformation of primary alcohols into alkenes catalysed by Mn(I)-PNP complexes consists of two cycles. First, the acceptorless dehydrogenation of the alcohol into aldehyde is produced via a concerted mechanism. Secondly, in an excess of hydrazine, hydrazone is formed and reacts with the aldehyde to produce olefins. This process, taking place in base-free conditions, is characterised by the diastereoselective formation of diazenyl intermediates. Based on DFT data, the generation of the (SN,S,S) diastereoisomer is favoured over the rest, leading in its decomposition to the preferential formation of an (E)-alkene and liberating N2 and H2O as the only by-products.
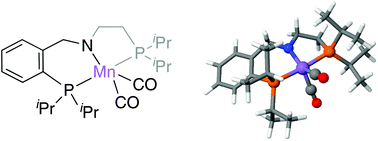


 Please wait while we load your content...
Please wait while we load your content...