Phosphine-functionalised tris(pyrazolyl)methane ligands and their mono- and heterobimetallic complexes†‡
Abstract
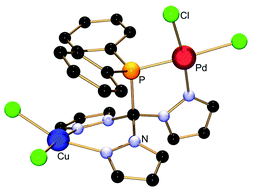
The synthesis, characterisation and reactivity of new phosphine-functionalised tris(pyrazolyl)methane ligands (TpmPR2, 2a–c, with R = Ph, nBu, iPr) are presented. The reaction of 2a–c with [Rh(CO)2Cl]2 furnished N,P-heterochelate carbonyl complexes (3a–c), which were used to quantify the donor abilities of the ligands via IR spectroscopy. The coordination flexibility was demonstrated by treating representative members of this new ligand class with [CpRu(acn)3][PF6] (acn = acetonitrile), [(tht)AuCl] (tht = tetrahydrothiophene) and [Pd(allyl)Cl]2 providing either a N,N,P-heteroscorpionate complex (4) or the P-coordinated complexes (5,6) without any involvement of the pyrazolyl entities. With 2a and [Pd(cod)Cl2], another P,N-heterochelate complex (7) was obtained, which served as a precursor for a heterobimetallic complex containing palladium and copper (8). Detailed NMR spectroscopic and X-ray crystallographic investigations have been performed on all new complexes.
- This article is part of the themed collection: Modern coordination chemistry


 Please wait while we load your content...
Please wait while we load your content...