Reactions of hydrazones and hydrazides with Lewis acidic boranes†
Abstract
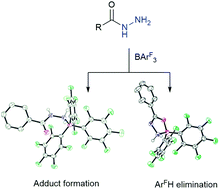
The reaction of (diphenylmethylene)hydrazone or 1,4-bis-hydrazone-ylidene(phenylmethyl)benzene with Lewis acidic boranes B(2,4,6-F3C6H2)3 or B(3,4,5-F3C6H2)3 generates the Lewis acid–base adducts. Alternatively, when (9H-fluoren-9-ylidene)hydrazone is employed several products were isolated including 1,2-di(9H-fluoren-9-ylidene)hydrazone, the 2 : 1 borane adduct of NH2–NH2 and the 1-(diarylboraneyl)-2-(9H-fluoren-9-ylidene)hydrazone in which one ArH group has been eliminated. The benzhydrazide starting material also initially gives an adduct when reacted with Lewis acidic boranes which upon heating eliminates ArH generating a CON2B heterocycle.


 Please wait while we load your content...
Please wait while we load your content...