The effect of locking π-conjugation in organoboron moieties in the structures of luminescent tetracoordinate boron complexes†
Abstract
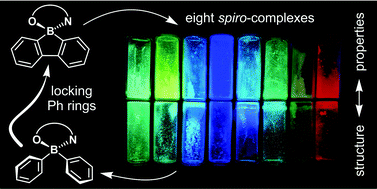
A series of 8 luminescent borafluorene complexes were extensively studied both experimentally and theoretically in order to elucidate the effect of organoboron moiety rigidification on the physicochemical properties of these compounds. Due to the spiro geometry of the boron atom, borafluorene and ligand units are perpendicularly aligned, which considerably affects the flexibility of the molecule as well as its solid-state structure. Through comparative analysis with close diphenyl analogues, we show how these structural features influence the thermal, photoluminescent and charge mobility behaviour of the studied compounds. Crystal structural analysis revealed that the molecules are connected mostly through C–H⋯O and C–H⋯ π interactions formed between perpendicularly aligned borafluorene and ligand moieties from neighboured molecules, serving as a complementary donor and acceptor of electron density, respectively. This also efficiently prevents molecules from engaging in unfavoured π-stacking contact. Furthermore, structural analysis suggests that borafluorene complexes possess a considerable degree of flexibility due to OBN heterocycle distortions and mutual borafluorene-ligand plane movements. The magnitude of these effects strictly depends on the ligand structure and may lead either to enhancing or lowering the quantum yield value with respect to BPh2 analogues, while the absorption and emission wavelength are slightly affected. The measured photophysical parameters for solid-state samples showed that the studied complexes are much better emitters in their crystalline states that in amorphous films. The TD-DFT and NTO calculations revealed a significant change in frontier molecular distribution, with the HOMO localized on the borafluorene moiety. However, as the HOMO–LUMO transition is geometrically not favoured, excitation occurred from HOMO−1 localized on the ligand. Finally, aggregation effects were discussed based on supramolecular arrangements in crystal structures and charge transfer rates obtained from theoretical calculations in the framework of the Marcus–Hush approximation. They suggest that borafluorene complexes are much better electron carriers with respect to non-annulated BPh2 complexes.


 Please wait while we load your content...
Please wait while we load your content...