Revisiting the reactivity of tetrachloroauric acid with N,N-bidentate ligands: structural and spectroscopic insights†
Abstract
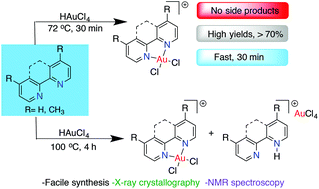
The reactivity of tetrachloroauric acid (HAuCl4) with readily accessible bidentate N-donor ligands affords N,N-ligated Au(III) center complexes. These compounds are useful precursors of stable catalysts, anticancer agents, and building blocks for materials. This report provides detailed insight into intermediates, equilibria, the counter anion effect, and structural variability, using spectroscopy, crystallography and computational tools. Novel mixed-valence Au(I) and Au(III) complexes [Au(o-phen)Cl2]2[AuCl2][AuCl4] and [Au(o-phen)Cl2][AuCl2] having AuCl2− and AuCl4− anions linearly arranged in the axial sites of the square-planar Au(o-phen)Cl2 cation were discovered. Other competing side products of the reaction studied revealed protonated N,N-bidentate ligands with AuCl4− anions. Quantitative variable temperature NMR studies reveal that for a mixture of target Au(III) salt and the protonated ligand, the reaction favors the irreversible formation of the side product. Using a rapid (30 min) temperature controlled protocol, the desired coordinated species is accessible in respectable yields while avoiding side products.


 Please wait while we load your content...
Please wait while we load your content...