Collective action of water molecules in zeolite dealumination†
Abstract
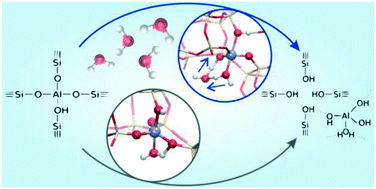
When exposed to steam, zeolite catalysts are irreversibly deactivated by loss of acidity and framework degradation caused by dealumination. Steaming typically occurs at elevated temperatures, making it challenging to investigate the mechanism with most approaches. Herein, we follow the dynamics of zeolite dealumination in situ, in the presence of a realistic loading of water molecules by means of enhanced sampling molecular dynamics simulations. H-SSZ-13 zeolite is chosen as a target system. Monte Carlo simulations predict a loading of more than 3 water molecules per unit cell at representative steaming conditions (450 °C, 1 bar steam). Our results show that a higher water loading lowers the free energy barrier of dealumination, as water molecules cooperate to facilitate hydrolysis of Al–O bonds. We find free energies of activation for dealumination that agree well with the available experimental measurements. Clearly, the use of enhanced sampling molecular dynamics yields a major step forward in the molecular level understanding of the dealumination; insight which is very hard to derive experimentally.


 Please wait while we load your content...
Please wait while we load your content...