Inductive and electrostatic effects on cobalt porphyrins for heterogeneous electrocatalytic carbon dioxide reduction†
Abstract
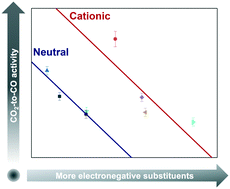
Electrochemical carbon dioxide reduction enables conversion of carbon dioxide into fuels and chemicals with renewable energy input. Cobalt-based molecular complexes have exhibited high selectivity, activity, and stability for transforming carbon dioxide into carbon monoxide. Through evaluating immobilized cobalt porphyrins functionalized with various peripheral substituents, we demonstrated that their activity is affected not only by the electronegativity of the substituents, but importantly, also by the charge of the substituents. The performance of immobilized cobalt porphyrins can be improved by introducing electron-donating and positively charged functional groups. Through kinetic studies, we were able to understand the mechanism by which electron-donating groups enhance the observed rates of carbon dioxide reduction and how cationic functionality may contribute towards electrostatic stabilization of the intermediate formed in the rate-determining step. Our methodology provides a robust and experimentally-verified method of computationally predicting the electronic effect of peripheral substitution and hence the catalytic activity of substituted porphyrins.
- This article is part of the themed collection: Catalysis for sustainable development


 Please wait while we load your content...
Please wait while we load your content...