Gelation enabled charge separation following visible light excitation using self-assembled perylene bisimides†
Abstract
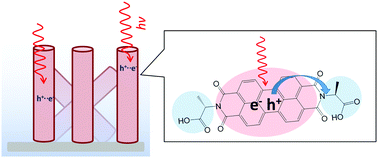
Perylene bisimides (PBIs) can be functionalised to enable controlled aggregation into complex supramolecular structures and are promising materials for photovoltaic and solar fuel applications. Amino acid appended PBIs such as PBI-alanine (PBI-A) have been found to form photoconductive films containing worm-like structures that enable charge transport. However, despite being strong chromophores in the visible region, when PBI-A films are prepared by drying down solutions, activity only occurs under UV illumination. This limits potential applications. The requirement for UV illumination has previously been suggested to be due to the large ion-pair energy in the low dielectric environment of the dried samples. Hydrogel films, rehydrated xerogels and dry xerogels of PBI-A can also be prepared offering an ideal sample set to examine the influence of hydration on charge-separation. Using transient absorption (TA) spectroscopy, we demonstrate a correlation between water content and efficiency of generation of long-lived charge separated states within the PBI-A materials, highlighting their potential, particularly for light-driven water splitting.


 Please wait while we load your content...
Please wait while we load your content...