Excited state C–N bond dissociation and cyclization of tri-aryl amine-based OLED materials: a theoretical investigation†
Abstract
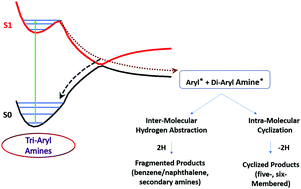
Tri-aryl amines (TAA) such as triphenylamine (TPA) are widely used for designing chromophores for dye-sensitized solar cells (DSSC) and organic light-emitting diodes (OLED). These materials degrade over time and hence result in reduced performance. Therefore, exploring the associated mechanistic pathways and factors controlling the degradation is necessary for future development of durable TAA-based devices. Hence, in this study, the complete active space self-consistent-field (CASSCF) method coupled with second order N-electron valence perturbation theory (NEVPT2) calculations was carried out to understand the excited state phenomena occurring in TAA using TPA and N,N-diphenyl-2-naphthylamine (DPNA) as model systems. The results indicated the presence of a conical intersection between ground and first excited singlet states with C–N bond dissociation, which acts as a channel for the excited molecules to dissociate and form radical fragments (phenyl/naphthyl). This occurrence is unusual for non-saturated bonds with delocalization. The resulting radical fragments formed intra-molecular products and subsequently yielded five- and six-membered cyclized products depending on the type of aryl groups. The significant findings from this study throw light on the photostability of TAA-based OLED devices as well as on the possible route to synthesize cyclic amines such as carbazoles.


 Please wait while we load your content...
Please wait while we load your content...