Ion speciation: a key for the understanding of the solution properties of ionic liquid mixtures†
Abstract
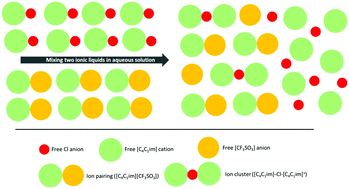
Recently, combinations of two (or more) ionic liquids, known as ionic liquid mixtures, have become popular and have a broad range of applications. However, the fundamental knowledge on the molecular interactions that exist in ionic liquid mixtures is far from being understood. In this work, the experimental measurement of the water activity coefficient and computational modelling using Conductor-like Screening Model for Real Solvent (COSMO-RS) were carried out to get an insight into the molecular interactions that are present in ionic liquid mixtures in aqueous solution. The results show that the combination of two ionic liquids of different basicity in aqueous solution allows fine tuning of the water activities, covering a wide range of values that could replace several pure fluids. This is an important feature resulting from the unexpected ion speciation of the ionic liquid mixtures in aqueous solution.


 Please wait while we load your content...
Please wait while we load your content...