Redox processes in sodium vanadium phosphate cathodes – insights from operando magnetometry†
Abstract
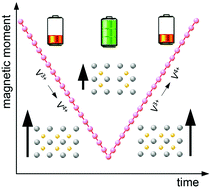
Operando magnetic susceptibility measurements of sodium ion cathode materials during repetitive electrochemical cycling enable a continuous and bulk sensitive monitoring of the transition metal oxidation states. Such measurements on NaxV2(PO4)3 identified vanadium to be the only ion undergoing oxidation/reduction processes upon battery operation. For the initial battery charging–discharging cycle as well as for the first cycle after prolonged room temperature storage, however, peculiarities within the magnetic susceptibility measurements indicate parasitic side reactions, likely on the cathode surface.


 Please wait while we load your content...
Please wait while we load your content...