Effect of atomicity on the oxidation of cationic copper clusters studied using thermal desorption spectrometry†
Abstract
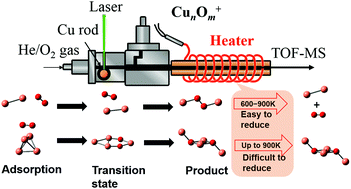
The resistivity to oxidation of small copper clusters, Cun+ (n ≤ 5), in the gas phase with a precise atomicity at the molecular level was investigated using a combination of thermal desorption spectrometry and mass spectrometry. Oxide clusters, CunOm+, with more O atoms than those present with a stoichiometry of n : m = 1 : 1 were produced at room temperature in the presence of O2, and the weakly bound excess oxygen atoms involved in the clusters were removed by post heating. Non-oxidized Cu2+ and Cu3+ clusters were formed in the range of 323–923 K, whereas partially oxidized clusters, Cu4O2+ and Cu5O2+, were generated for n = 4 and 5. Considering the fact that CunOm+ (m = n/2 + 1) tends to be generated for n ≥ 6, the small copper clusters were concluded to be resistive to oxidation. The possible reaction paths for the oxidation of Cu2+ and Cu4+ clusters were obtained by density functional calculations, which were consistent with the experimental findings. The oxidation states of the Cu atoms in the clusters were discussed based on the natural charges of the atoms.


 Please wait while we load your content...
Please wait while we load your content...