Soft experimental constraints for soft interactions: a spectroscopic benchmark data set for weak and strong hydrogen bonds†
Abstract
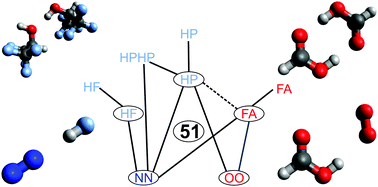
An experimental benchmark data base on rotational constants, vibrational properties and energy differences for weakly and more strongly hydrogen-bonded complexes and their constituents from the spectroscopic literature is assembled. It is characterized in detail and finally contracted to a more compact, discriminatory set (ENCH-51, for Experimental Non-Covalent Harmonic with 51 entries). The meeting points between theory and experiment consist of equilibrium rotational constants and harmonic frequencies and energies, which are back-corrected from experimental observables and are very easily accessible by quantum chemical calculations. The relative performance of B3LYP-D3, PBE0-D3 and M06-2X density functional theory predictions with a quadruple-zeta basis set is used to illustrate systematic errors, error compensation and selective performance for structural, vibrational and energetical observables. The current focus is on perspectives and different benchmarking methodologies, rather than on a specific theoretical method or a specific class of compounds. Extension of the data base in chemical, observable and quantum chemical method space is encouraged.


 Please wait while we load your content...
Please wait while we load your content...