Molten alkali halides – temperature dependence of structure, dynamics and thermodynamics†
Abstract
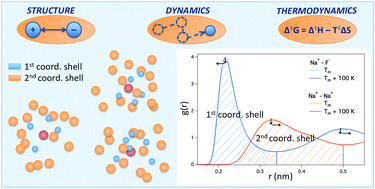
The renewed interest in molten salts in the energy industry fuels the need of a thorough understanding of their physicochemical properties. Alkali halide melts are perhaps the simplest ionic liquids, but they are used as electrolytes in batteries or for thermal energy storage. Although their structure is considered to be well documented and understood, a systematic evaluation of experimental structural data reveals significant discrepancies, while there is only limited experimental information on dynamic properties. Here, we investigate structure, dynamics and thermodynamic properties of pure alkali halide melts using state-of-the-art simulation models at different temperatures. The simulations provide a consistent picture of the structure of alkali halide melts with coordination numbers that lie in between experimental numbers. The simulations reveal a strengthening of the cation–anion bonds with increasing temperature that, somewhat counter-intuitively, coincides with faster dynamics in the melts. The thermodynamic analysis unveils that structure breaking proceeds on the picosecond timescale through an associative substitution mechanism as signified by a negative entropy of activation. The results on ion pair lifetimes contribute to an improved understanding of the microscopic origin of dynamical properties, such as e.g. conductivity of salt melts. The structural analysis provided here contributes to a more coherent picture of the coordination numbers in alkali halides than what is currently available from experimental data.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...