Effect of confinement on the adsorption behavior of inorganic and organic ions at aqueous–cyclohexane interfaces: a molecular dynamics study†
Abstract
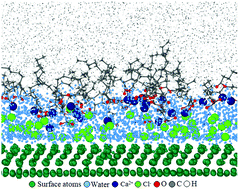
Molecular dynamics simulation was used to study the adsorption behavior of inorganic and organic ions at aqueous–cyclohexane interfaces, and that with such systems confined in a kaolinite nanopore. Four aqueous solutions were used, each containing one of the four solutes (NaCl, NaOH, CaCl2 and Ca(OH)2) at the concentration of 1.0 M. At the interface of each of the solutions with neat cyclohexane, there existed an ion depletion zone. The more strongly hydrated ions, such as calcium and hydroxide, were more intensely depleted from the interface as compared to sodium and chloride. Such surface exclusion led to interfacial tension increases, with greater increments for solutions containing calcium or hydroxide. Upon addition of sodium decanoate to the cyclohexane phase, they partly migrated to the aqueous–organic interface, and the remaining formed inverted micelle complexes. Also, a small fraction of the solvated cations in the aqueous phase drifted to the depletion zone to interact with the organic anions, with their affinity towards the interface still being controlled by their hydration-strength. When such systems were confined in a kaolinite nanopore, behavior of decanoate anions – which were found to be prone to strong adsorption to solid surfaces in the absence of any aqueous solution – was determined by the nature of the solvated ions in water. In these systems, the more weakly-hydrated ionic species exhibited preferential adsorption to the solid surface, while affinity of the more strongly-hydrated ones was towards remaining within the water layers or to the aqueous–organic interface. With the calcium chloride solution, almost all of the organic ions were detached from the surface and adsorbed at the aqueous–cyclohexane interface. This was caused by the release of double amount of inorganic anions by this 1 : 2 salt, the inner-sphere adsorption of majority of chloride anions to the octahedral surface, and the higher charge density of calcium cations.


 Please wait while we load your content...
Please wait while we load your content...