How to add a five-membered ring to polycyclic aromatic hydrocarbons (PAHs) – molecular mass growth of the 2-naphthyl radical (C10H7) to benzindenes (C13H10) as a case study†
Abstract
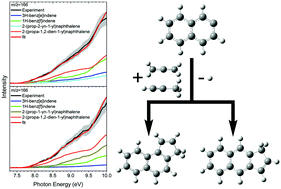
The three-ring polycyclic aromatic hydrocarbons (PAHs) 3H-benz[e]indene (C13H10) and 1H-benz[f]indene (C13H10) along with their naphthalene-based isomers 2-(prop-2-yn-1-yl)naphthalene (C13H10), 2-(prop-1-yn-1-yl)naphthalene (C13H10), and 2-(propa-1,2-dien-1-yl)naphthalene (C13H10) were formed through a “directed synthesis” via a high temperature chemical micro reactor under combustion-like conditions (1300 ± 35 K) through the reactions of the 2-naphthyl isomer (C10H7˙) with allene (C3H4) and methylacetylene (C3H4). The isomer distributions were probed utilizing tunable vacuum ultraviolet radiation from the Advanced Light Source (ALS) by recording the photoionization efficiency curves at mass-to-charge of m/z = 166 (C13H10) and 167 (13CC12H10) of the products in a supersonic molecular beam. Complemented by electronic structure calculations, our study reveals critical mass growth processes via annulation of a five-membered ring from the reaction between aryl radicals and distinct C3H4 isomers at elevated temperatures as present in combustion processes and in circumstellar envelopes of carbon stars. The underlying reaction mechanisms proceed through the initial addition of the 2-naphthyl radical with its radical center to the π-electron density of the allene and methylacetylene reactants via entrance barriers between 8 and 14 kJ mol−1, followed by isomerization (hydrogen shifts, ring closure), and termination via atomic hydrogen losses accompanied by aromatization. The reaction mechanisms reflect the formation of indene – the prototype PAH carrying a single five- and a single six-membered ring – synthesized through the reaction of the phenyl radical (C6H5˙) with allene and methylacetylene. This leads us to predict that aryl radicals – upon reaction with allene/methylacetylene – may undergo molecular mass growth processes via ring annulation and de facto addition of a five-membered ring to form molecular building blocks essential to transit planar PAHs out of the plane.


 Please wait while we load your content...
Please wait while we load your content...
