Delayed photoacidity produced through the triplet–triplet annihilation of a neutral pyranine derivative†
Abstract
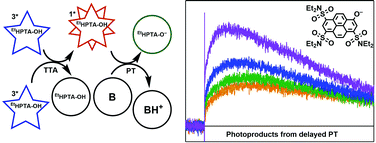
A novel pyranine derivative, EtHPTA-OH, was synthesized via the substitution of the anionic sulfonate groups with neutral diethylsulfonamide groups. The photophysical and photochemical properties of EtHPTA-OH were studied using photoluminescence quenching and transient absorption spectroscopy. The singlet state of EtHPTA-OH was found to be highly photoacidic (pKa* = 8.74 in acetonitrile). A series of aniline and pyridine bases were used to investigate excited-state proton transfer (ESPT) from singlet EtHPTA-OH, and rate constants for singlet quenching via ESPT were determined (kq = 5.18 × 109 to 1.05 × 1010 M−1 s−1). EtHPTA-OH was also found to exhibit a long-lived triplet state which reacts through a triplet–triplet annihilation (TTA) process to reform singlet EtHPTA-OH on timescales of up to 80 μs. Detection of ESPT photoproducts on timescales comparable to that of TTA singlet regeneration provides strong evidence for photoacidic behavior stemming from the regenerated singlet EtHPTA-OH.


 Please wait while we load your content...
Please wait while we load your content...
