Hydrogen tunneling avoided: enol-formation from a charge-tagged phenyl pyruvic acid derivative evidenced by tandem-MS, IR ion spectroscopy and theory†
Abstract
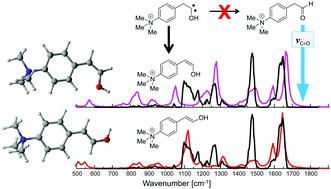
A charge-tagged phenyl pyruvic acid derivative was investigated by tandem-MS, infrared (IR) ion spectroscopy and theory. The tailor-made precursor ions efficiently lose CO2 in collision induced dissociation (CID) experiments, offering access to study the secondary decay reactions of the product ions. IR ion spectroscopy provides evidence for the formation of an enol acid precursor ion structure in the gas phase and indicates the presence of enol products formed after CO2 loss. Extensive DFT computations however, suggest intermediate generation of hydroxycarbene products, which in turn rearrange in a secondary process to the enol ions detected by IR ion spectroscopy. Quantum mechanical tunneling of the hydroxycarbene can be excluded since no evidence for aldehyde product ion formation could be found. This finding is in contrast to the behavior of methylhydroxycarbene, which cleanly penetrates the energy barrier to form exclusively acetaldehyde at cryogenic temperatures in an argon matrix via quantum mechanical hydrogen tunneling. The results presented here are attributed to the highly excited energy levels of the product ions formed by CID in combination with different barrier heights of the competing reaction channels, which allow exclusive access over one energy barrier leading to the formation of the enol tautomer ions observed.


 Please wait while we load your content...
Please wait while we load your content...