Site-specific X-ray induced dynamics in liquid methanol†
Abstract
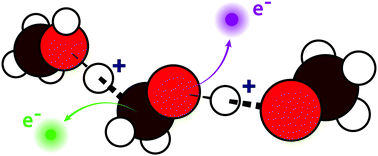
Complex chemical and biochemical systems are susceptible to damage from ionising radiation. However, questions remain over the extent to which such damage is influenced by the nature of the surrounding chemical environment, which can consist of both hydrophobic and hydrophilic domains. To gain fundamental insight into the first crucial mechanistic steps of radiation damage in such systems, we need to understand the initial radiation response, i.e. dynamics occurring on the same timescale as electronic relaxation, which occur in these different environments. Amphiphilic molecules contain both hydrophobic and hydrophilic domains, but the propensity for charge delocalisation and proton dynamics to occur in these different domains has been largely unexplored so far. Here, we present carbon and oxygen 1s Auger spectra for liquid methanol, one of the simplest amphiphilic molecules, as well as its fully deuterated equivalent d4-methanol, in order to explore X-ray induced charge delocalisation and proton dynamics occurring on the few femtosecond timescale. Unexpectedly, we find a similar propensity for proton dynamics to occur at both the carbon and oxygen site within the lifetime of the core hole. Our results could serve as a model for decay processes that are likely to occur in other more complex amphiphilic systems.


 Please wait while we load your content...
Please wait while we load your content...