Theoretical insights into inorganic–organic intercalation products of the layered perovskite HLaNb2O7: perspectives for hybrid proton conductors†
Abstract
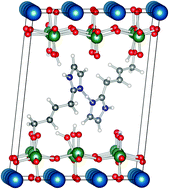
The modification of metal oxide surfaces with organic moieties has been widely studied as a method of preparing organic–inorganic hybrid materials for various applications. Among the inorganic oxides, ion-exchangeable layered perovskites are particularly interesting, because of their appealing electronic and reactive properties. In particular, their protonated interlayer surface can be easily functionalized with organic groups allowing the production of stable hybrid materials. As a further step in the design of new inorganic–organic hybrid proton conductors, a combined experimental and theoretical study of two intercalated compounds (propanol and imidazole) in HLaNb2O7 is presented here. A generally very good agreement with the available experimental data is found in reproducing both structural features and 13C-NMR chemical shifts, and marked differences between the two considered intercalated compounds are evidenced, with possible important outcomes for proton conduction. Notably, the free imidazole molecules are easily protonated by the acidic protons present in the interlayer spacing, thus inhibiting an efficient charge transport mechanism. In order to overcome this problem, a model system has been considered, where the imidazoles are bound to the end of a butyl chain, the whole being intercalated between two perovskite layers. The obtained theoretical data suggest that, in such a system, proton transfer between two adjacent imidazoles is a barrierless process. These results could then open new perspectives for such hybrid proton conductors.


 Please wait while we load your content...
Please wait while we load your content...