Block copolymers as bile salt sequestrants: intriguing structures formed in a mixture of an oppositely charged amphiphilic block copolymer and bile salt†
Abstract
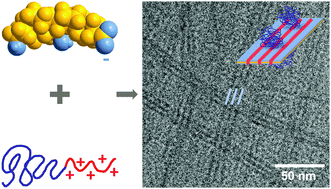
To study the formation and characterize the structure of mixed complexes of oppositely charged block copolymers and surfactants are of great significance for practical applications, e.g., in drug carrier formulations that are based on electrostatically assisted assembly. In this context, biocompatible block copolymers and biosurfactants (like bile salts) are particularly interesting. In this work, we report on the co-assembly in dilute aqueous solution between a cationic poly(N-isopropyl acryl amide) (PNIPAM) diblock copolymer and the oppositely charged bile salt surfactant sodium deoxycholate at ambient temperature. The cryogenic transmission electron microscopy (cryo-TEM) experiments revealed the co-existence of two types of co-assembled complexes of radically different morphology and inner structure. They are formed mainly as a result of the electrostatic attraction between the positively charged copolymer blocks and bile salt anions and highlight the potential of using linear amphiphilic block copolymers as bile salt sequestrants in the treatment of bile acid malabsorption and hypercholesterolemia. The first complex of globular morphology has a coacervate core of deoxycholate anions and charged copolymer blocks surrounded by a PNIPAM corona. The second complex has an intriguing tape-like supramolecular morphology of several micrometer in length that is striped in the direction of the long axis. A model is presented in which the stretched cationic blocks of several block copolymers interact electrostatically with the bile salt molecules that are associated to form a zipper-like structure. The tape is covered on both sides by the PNIPAM chains that stabilize the overall complex in solution. In addition to cryo-TEM, the mixed system was investigated in a range of molar charge fractions at a constant copolymer concentration by static light scattering, small angle X-ray scattering, and electrophoretic mobility measurements.


 Please wait while we load your content...
Please wait while we load your content...