Ultrafast photoisomerisation of an isolated retinoid†
Abstract
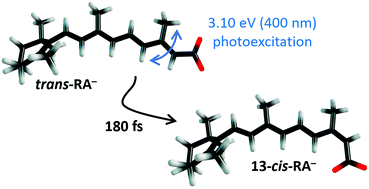
The photoinduced excited state dynamics of gas-phase trans-retinoate (deprotonated trans-retinoic acid, trans-RA−) are studied using tandem ion mobility spectrometry coupled with laser spectroscopy, and frequency-, angle- and time-resolved photoelectron imaging. Photoexcitation of the bright S3(ππ*) ← S0 transition leads to internal conversion to the S1(ππ*) state on a ≈80 fs timescale followed by recovery of S0 and concomitant isomerisation to give the 13-cis (major) and 9-cis (minor) photoisomers on a ≈180 fs timescale. The sub-200 fs stereoselective photoisomerisation parallels that for the retinal protonated Schiff base chromophore in bacteriorhodopsin. Measurements on trans-RA− in methanol using the solution photoisomerisation action spectroscopy technique show that 13-cis-RA− is also the principal photoisomer, although the 13-cis and 9-cis photoisomers are formed with an inverted branching ratio with photon energy in methanol when compared with the gas phase, presumably due to solvent-induced modification of potential energy surfaces and inhibition of electron detachment processes. Comparison of the gas-phase time-resolved data with transient absorption spectroscopy measurements on retinoic acid in methanol suggest that photoisomerisation is roughly six times slower in solution. This work provides clear evidence that solvation significantly affects the photoisomerisation dynamics of retinoid molecules.


 Please wait while we load your content...
Please wait while we load your content...