Interaction of the simple carbene c-C3H2 with H2: potential energy surface and low-energy scattering
Abstract
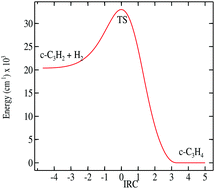
Cyclopropenylidene, c-C3H2, is a simple hydrocarbon, ubiquitous in astrophysical gases, and possessing a permanent electric dipole moment. Its readily observed rotational transitions make it an excellent probe for the physics and history of interstellar matter. The collisional properties of c-C3H2 with the main background gas, H2, are computed here. We present a full 5-D Potential Energy Surface in the rigid molecule approach, and fit it to relevant functionals for subsequent scattering. We perform low-energy quantum scattering at energies less than 50 cm−1. We use both ortho and para H2 as projectiles. We determine the quality of the various approximations to exact coupled channel scattering and examine paths to go towards higher energy scattering relevant for astrophysics. We compare the results obtained here with earlier ones for scattering with helium.


 Please wait while we load your content...
Please wait while we load your content...