On the linear geometry of lanthanide hydroxide (Ln-OH, Ln = La–Lu)†
Abstract
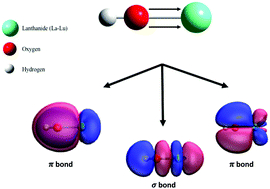
Lanthanide hydroxides are key species in a variety of catalytic processes and in the preparation of corresponding oxides. This work explores the fundamental structure and bonding of the simplest lanthanide hydroxide, LnOH (Ln = La–Lu), using density functional theory calculations. Interestingly, the calculations predict that all structures of this series will be linear. Furthermore, these results indicate a valence electron configuration of σ2π4 for all LnOH compounds, suggesting that the lanthanide-hydroxide bond is best characterized as a covalent triple bond.


 Please wait while we load your content...
Please wait while we load your content...