Thermodynamics and reaction mechanism of urea decomposition†
Abstract
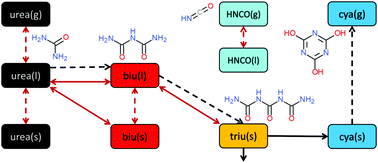
The selective catalytic reduction technique for automotive applications depends on ammonia production from a urea–water solution via thermolysis and hydrolysis. In this process, undesired liquid and solid by-products are formed in the exhaust pipe. The formation and decomposition of these by-products have been studied by thermogravimetric analysis and differential scanning calorimetry. A new reaction scheme is proposed that emphasizes the role of thermodynamic equilibrium of the reactants in liquid and solid phases. Thermodynamic data for triuret have been refined. The observed phenomenon of liquefaction and re-solidification of biuret in the temperature range of 193–230 °C is explained by formation of a eutectic mixture with urea.


 Please wait while we load your content...
Please wait while we load your content...