Alkali carbonates promote CO2 capture by sodium orthosilicate
Abstract
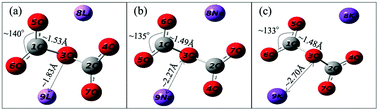
Sodium orthosilicate was synthesized by a wet chemical method with further calcination at 600 °C. Mixtures of Na4SiO4 and alkali (Li/Na/K) carbonates were prepared by a mechanical mixing method. The CO2 capture performance of the samples was characterized by dynamic thermogravimetric analyses and in situ XRD and Raman spectroscopy in 80 vol% CO2 mixed with 20 vol% N2. It was found that sodium orthosilicate could be used for CO2 sorption, and its maximum capacity could reach up to 19.2 wt%. The addition of alkali carbonates, which serve as promoters, led to the enhancement of the CO2 capture performance of Na4SiO4, especially at low temperatures, because of the formation of C2O52−. The existence of C2O52− in the mixture exposed to 80 vol% CO2 was confirmed by in situ Raman spectra, and its geometric structure was presented by DFT calculations. The formation of C2O52− within carbonates exhibited a positive influence on the CO2 capture at low temperatures and the enhancement of CO2 diffusion by Grotthuss-like transport through the carbonate product shell at high temperatures besides the formation of eutectic carbonate melts.


 Please wait while we load your content...
Please wait while we load your content...