Calculation of apparent pKa values of saturated fatty acids with different lengths in DOPC phospholipid bilayers†
Abstract
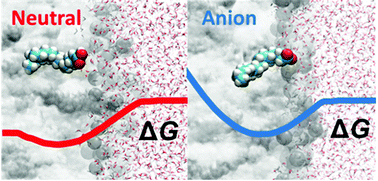
We performed all-atom molecular dynamics simulations and calculated free energy profiles and apparent pKa values for neutral and anionic forms of single myristic (C14:0), palmitic (C16:0) and stearic (C18:0) fatty acid embedded in a DOPC bilayer and explicit water solvent. We showed that the neutral forms of the fatty acids are stabilized inside the bilayer by hydrogen bonding of a fatty acid carboxylic group with DOPC phosphate and carbonyl groups. In contrast to the neutral form, the anionic forms of the fatty acids are shifted towards the water–membrane interface and are instead stabilized by hydrogen bonding to interfacial water. By using umbrella sampling simulations, we calculated free energies of stabilization and revealed that the free energy of stabilization inside the bilayer increases with the chain length for both the neutral and deprotonated forms. On the other hand, the free energies of flip-flop of both the neutral and anionic forms are constant upon the prolongation of the fatty acid. Based on the free energy curves, we also calculated apparent fatty acid pKa,app values in the bilayer, which are 7.0, 7.2 and 6.3 for myristic, palmitic and stearic acid and are increased by several pKa units compared to the corresponding pKa values in water. By further analysis of the calculated curves we found that spontaneous protonation of fatty acid anions takes place in the bilayer interior at ca. 1.4 nm from the bilayer center for all studied fatty acids.


 Please wait while we load your content...
Please wait while we load your content...