Does aromaticity account for an enhanced thermodynamic stability? The case of monosubstituted azaborines and the stereoelectronic chameleonism of the NH2 group†
Abstract
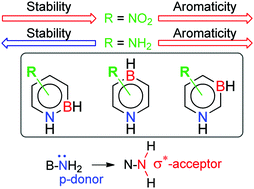
This work was initiated by the increasing interest in BN/CC isosterism and by the long-lasting interest in the concepts of aromaticity and substituent effects. We have theoretically examined the aromaticity and stability of monosubstituted BN isosters of benzene, the three isomeric azaborines. The results provide insight into the effect of substitution on two basic molecular properties, which are influenced, here, by the substituent effects and by the B/N relationship in the ring. The results, along with other examples in the literature, also warn chemists that the general belief that aromaticity accounts for enhanced thermodynamic stability is not always true. The stability of cyclic, conjugated compounds depends on several effects, and only one of them is aromaticity. In addition, our calculations predict a switching of electronic properties of the NH2 group from the usual p-electron donor to a π-electron acceptor when it is moved from the B/C atoms to the nitrogen atom in all isomers, or C6 in 1,3-azaborine. This is the result of the conformational change that places the NLP in the plane of the ring and the NH bonds in a favourable spatial position to act as acceptors of π-electron density.


 Please wait while we load your content...
Please wait while we load your content...