The synthesis and photophysical properties of tris-coumarins†
Abstract
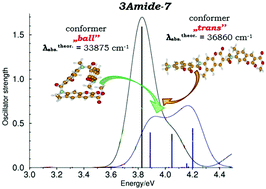
A structurally unique cyclic tris-coumarin possessing three identical coumarin units bridged by amide linkers as well as two linear analogs has been synthesized. There is a remarkable agreement between crystallographic data, 1H NMR and results of calculations for the cyclic tris-coumarin, showing in all cases a non-symmetric arrangement of identical coumarin moieties. Weak polarization of the coumarin subunits, resulting from the presence of only CONH– groups as electron-donors, results in a hypsochromic shift of both absorption and emission in this dye. We have proven that in non-cyclic, head-to-tail linked tris-coumarins, the photophysics is controlled not only by the substituents but also by the conformation of the molecule, which in turn depends on the nature of the linker's interactions. These can be controlled by the presence/absence of an amide-type hydrogen atom responsible for the formation of intramolecular hydrogen bonds. The presence of a hydrogen bond favors a stretched trans conformation of the dye, while in its absence, folding of the molecule occurs leading to a more compact conformation. Although, the increased number of covalently linked coumarin units does not drastically change the preferred conformation, the fluorescence quantum yields of tris-coumarins are significantly lower than for analogous bis-coumarins composed of the same units.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...