NASICON-type polymer-in-ceramic composite electrolytes for lithium batteries†
Abstract
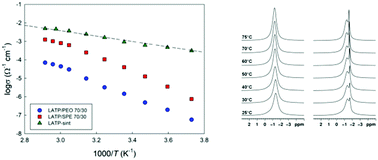
Hybrid polymer–ceramic electrolytes with high ceramic loading are currently investigated as a promising solution to achieve high safety and optimal mechanical properties in all-solid-state rechargeable batteries. In this study composite poly(ethylene oxide)/Li1.3Al0.3Ti1.7(PO4)3 (PEO/LATP) electrolytes, with and without lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) as the Li+ salt, were investigated through a combination of physicochemical and electrochemical techniques, including X-ray diffraction, scanning electron microscopy, thermal analysis, solid-state MAS-NMR and impedance spectroscopy. We were able to shed light on the interactions between the ceramic and the polymer phases, and on the mechanisms for Li+ transport. Membranes containing 70 wt% of LATP and 30 wt% of P(EO)15LiTFSI exhibit conductivity values of 4 × 10−5 Ω−1 cm−1 at 25 °C and in excess of 10−4 Ω−1 cm−1 at 45 °C. These promising results, obtained on a quasi-ceramic electrolyte through room temperature processing, suggest that further improvements in the transport properties of “polymer-in-ceramic” systems may be sought by increasing the amorphous polymer content, and by carefully investigating the role of the ceramic particles’ composition, dimensions and dispersion on the transport properties of the hybrid system.


 Please wait while we load your content...
Please wait while we load your content...