Accurate entropy calculation for large flexible hydrocarbons using a multi-structural 2-dimensional torsion method†
Abstract
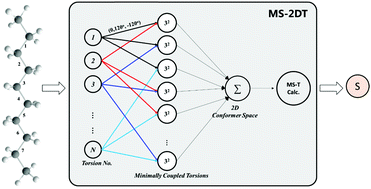
Entropy is one of the key thermodynamic parameters in combustion kinetic modeling. Accurate entropy prediction needs to account for the conformational torsional anharmonicity, which could be solved by the state-of-the-art multi-structural torsion (MS-T) method. However, this method is computationally expensive or even not feasible for large flexible molecules. To address this issue, we proposed a multi-structural 2-dimensional torsion (MS-2DT) method that adopts minimally coupled torsions to reduce the computational cost. In this method, a series of 2-dimensional coupled torsion combinations were used to generate an initial conformer space with a size of CN2·9 (N is the number of torsions). The standard entropy (and the heat capacity) values of 18 C6–C8 alkanes with 5–7 torsions were computed at 200–2000 K. The MS-2DT calculation is in good agreement with the benchmark MS-T method: only a small deviation of −0.19 ± 0.15 cal mol−1 K−1 in standard entropy and −0.10 ± 0.21 cal mol−1 K−1 in heat capacity. Additionally, a further application of MS-2DT to n-decane with 9 torsions implies an improved accuracy in entropy (and heat capacity) prediction compared to other conventional simplified treatments. This method provides an affordable and accurate solution to treat the conformational torsional anharmonicity of large flexible alkanes.


 Please wait while we load your content...
Please wait while we load your content...