Formation and interconversion of CCN and CNC radicals resulting from the radiation-induced decomposition of acetonitrile in solid noble gas matrices†
Abstract
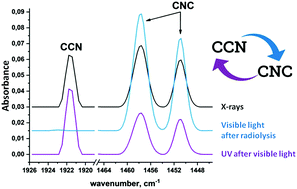
Acetonitrile and the species resulting from its dehydrogenation play an important role in the radiation-induced evolution of organic matter in the space environment. In this work, we report on FTIR spectroscopic studies of the degradation of isolated CH3CN and CD3CN molecules induced by prolonged X-ray irradiation in solid noble gas matrices at 5 K. The principal products observed at high conversion degree of the parent acetonitrile molecules (70–90%) are CCN and CNC radicals, which result from prompt or two-step dehydrogenation of the corresponding precursors, H2CN and CH2NC radicals, respectively. CHCN and CHNC were also found as products of dehydrogenation at high absorbed doses, whereas the fragmentation products (CH3, CN, HCN, and HNC) were detected only in minor amounts over the whole dose range studied. CCN and CNC are produced in nearly equal amounts at high absorbed doses. Selective isomerization of CCN to CNC was observed under the illumination with visible light (460–470 nm), while subsequent action of the UV light (254 nm) induced reverse transformation leading to a photostationary state with the relative population of CNC/CCN being ca. 0.7. The astrochemical implications of the obtained results are discussed in connection with the recent discovery of CCN in extraterrestrial objects.


 Please wait while we load your content...
Please wait while we load your content...