Macroscopic defects upon decomposition of CO2 clathrate hydrate crystals†
Abstract
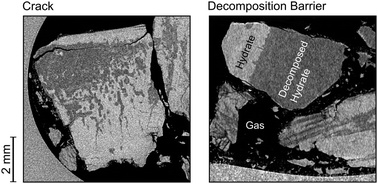
Micrometer- and submicrometer-sized pores and macroscopic defects like cracks and tubular channels can be found in a variety of clathrate hydrates (hydrates for short) during formation and decomposition. Their origin, their evolution in time, and their effect on hydrate decomposition kinetics are unclear. We used time-lapse micro computed tomography (μCT) in combination with temperature control and pressure monitoring to study the formation and evolution of pores and macroscopic defects in decomposing CO2 hydrates at subzero (Celsius) temperature. Our results suggest that the decomposition of hydrates is always accompanied by the formation of pores and an increase of the apparent hydrate volume by more than 3%. Hydrate decomposition often starts with the formation of cracks inside the hydrate and not necessarily at the free surface of the hydrate, which frequently remains intact for a long period and seems to be self-preserved in some regions. Decomposition spreads out from these cracks in a uniform fashion yielding a variety of macroscopic features. In some cases, the propagating decomposition front seems to get blocked by planar barriers inside the hydrate yielding regions with high resistance against decomposition. This, together with a generally heterogeneous distribution of decomposition resistant regions, challenges the shrinking core model of hydrate decomposition as well as the popular ice-rind theory used to explain the effect of self-preservation.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...