Exploring Norrish type I and type II reactions: an ab initio mechanistic study highlighting singlet-state mediated chemistry†
Abstract
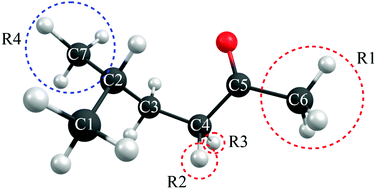
Norrish reactions are important photo-induced reactions in mainstream organic chemistry and are implicated in many industrially and biologically relevant processes and in the processing of carbonyl molecules in the atmosphere. The present study reports multi-reference electronic structure calculations designed to assess details of the potential energy profiles associated with the Norrish type-I and type-II reactions of a prototypical ketone 5-methyl-hexan-2-one. We show that the well-established ‘triplet state mediated’ reaction pathways following initial population of a singlet excited state can be complemented by (hitherto rarely recognized) ‘singlet state only’ Norrish type-I and type-II reaction mechanisms that involve no spin-forbidden transitions along the respective reaction paths, and suggest how the efficiencies of such reactions might be affected by strategic substitutions at selected sites within the parent ketone.
- This article is part of the themed collection: Photodissociation and reaction dynamics


 Please wait while we load your content...
Please wait while we load your content...