Density functional theory calculations and thermodynamic analysis of bridgmanite surface structure†
Abstract
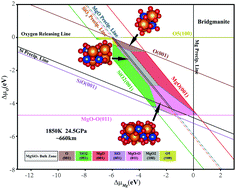
Bridgmanite, a high temperature and pressure form of MgSiO3, is believed to be Earth's most abundant mineral and responsible for the observed seismic anisotropy in the mantle. Little is known about surfaces of bridgmanite but knowledge of the most stable surface terminations is important for understanding various geochemical processes as well as likely slip planes. A density functional theory based thermodynamic approach is used here to establish the range of stability of bridgmanite as well as possible termination structures of the (001), (010), (100) and (011) surfaces as a function of the chemical potential of oxygen and magnesium. The vibrational contribution to the Gibbs free energy is found to be essential for obtaining a stability region of bridgmanite in the phase diagram. The most stable surface termination of bridgmanite varies between three different atomic structures depending on the chemical potential of oxygen and magnesium. The results presented provide a basis for further theoretical studies of the chemical processes on bridgmanite surfaces in the Earth's mantle and slip plane analysis.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...