Probing the gas-phase structure of charge-tagged intermediates of a proline catalyzed aldol reaction – vibrational spectroscopy distinguishes oxazolidinone from enamine species†
Abstract
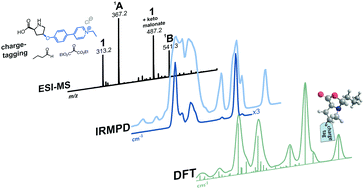
An L-proline based catalyst with a charged phenyl-pyridium substituent (1) was used to analyze intermediates of an organocatalyzed aldol reaction by infrared multi-photon dissociation (IRMPD) mass spectrometry after transfer into the gas phase via electrospray ionization (ESI). IRMPD spectra were interpreted with the aid of density functional theory (DFT) computations. A structurally restricted enamine species was used as a reference molecule for the calculated vibrational frequencies. A close correlation between theory and experiment was found for the energetically most favoured oxazolidinone structures.


 Please wait while we load your content...
Please wait while we load your content...