Growth of invar nanoparticles on a graphene oxide support†
Abstract
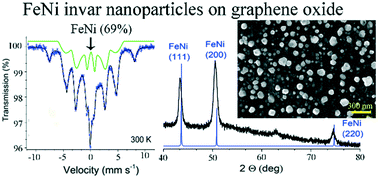
Binary nanoparticles, composed of two different metals, attract significant attention because they possess properties not typical for their respective single-component nanoparticles. In the bulk form, iron and nickel form an alloy called invar in which the two metals are mixed in a ratio of Fe : Ni = 2 : 1. In this work, we demonstrate the formation of alloyed nanoparticles of invar, as opposed to the theoretically possible formation of particles from the two individual metals. The formation of the alloyed nanoparticles is conducted in a two-step process: liquid phase impregnation of graphene oxide with the salts of the metals, and subsequent annealing of the as-formed dry composite. Unlike the solution phase reaction conditions, in this approach, the binary nanoparticles are assembled under conditions where the metal atoms are immobilized on the surface of the decomposing graphene oxide, and at temperatures significantly lower than the melting points of the two metals. The structure of the as-grown nanocrystals is investigated by Mössbauer spectroscopy, powder X-ray diffraction, X-ray photoelectron spectroscopy, and scanning electron microscopy. The majority of the Fe/Ni nanoparticles are in a magnetically ordered state, and the composite is a soft magnet.


 Please wait while we load your content...
Please wait while we load your content...