Construction of three-dimensional anionic molecular frameworks based on hydrogen-bonded metal dithiolene complexes and the crystal solvent effect†
Abstract
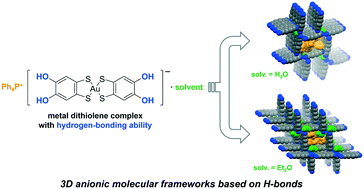
We have designed and synthesized a novel metal dithiolene complex with hydrogen-bonding (H-bonding) ability, [Au(catdt)2]− (catdt: catechol-4,5-dithiolate) and successfully constructed [Au(catdt)2]−-based three-dimensional (3D) anionic molecular frameworks in two kinds of salts, (Ph4P)[Au(catdt)2]·0.5H2O (3a) and (Ph4P)[Au(catdt)2]·Et2O·n(solv) (3b; solv = Et2O and/or acetone). To our knowledge, these are the first examples of the 3D molecular frameworks based on H-bonded metal dithiolene complexes. Importantly, these frameworks are constructed through 3D intermolecular H-bonds between [Au(catdt)2]− molecules, and have tubular channels occupied by large Ph4P+ cations, where multiple cation–anion short contacts are formed to stabilize the 3D framework structures. Furthermore, in addition to 3a and 3b with 3D frameworks, we have obtained (Ph4P)[Au(catdt)2]·2THF (3c) having a 2D layered structure. A detailed comparison of the structures of these compounds reveals that the included solvent molecules play an important role in regulating the intermolecular interactions and assembled structures. Interestingly, we have found that, due to the differences in the H-bonding ability and molecular size of H2O and Et2O, the wall structures of the 3D frameworks in 3a without voids and 3b with voids are qualitatively different.


 Please wait while we load your content...
Please wait while we load your content...