Determining isoleucine side-chain rotamer-sampling in proteins from 13C chemical shift†
Abstract
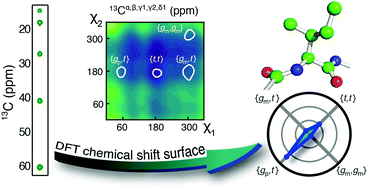
Chemical shifts are often the only nuclear magnetic resonance parameter that can be obtained for challenging macromolecular systems. Here we present a framework to derive the conformational sampling of isoleucine side chains from 13C chemical shifts and demonstrate that side-chain conformations in a low-populated folding intermediate can be determined.


 Please wait while we load your content...
Please wait while we load your content...