Continuous flow knitting of a triptycene hypercrosslinked polymer†
Abstract
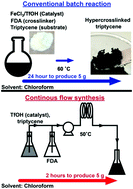
By replacing Lewis acids with Brønsted acids as catalysts, continuous flow synthesis of hypercrosslinked polymers is achieved within 10% of the time required for a typical batch reaction. Compared with batch-synthesised polymers, the flow-produced materials take up 24% more CO2, precluding the need for lengthy reaction protocols to yield high-performance hypercrosslinked polymers for carbon capture.


 Please wait while we load your content...
Please wait while we load your content...