Use of pyridazinediones as extracellular cleavable linkers through reversible cysteine conjugation†
Abstract
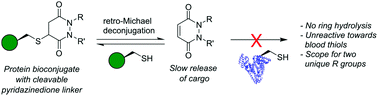
Herein we report a retro-Michael deconjugation pathway of thiol–pyridazinedione linked protein bioconjugates to provide a novel cleavable linker technology. We demonstrate that the novel pyridazinedione linker does not suffer from off-target modification with blood thiols (e.g., glutathione, human serum albumin (HSA)), which is in sharp contrast to an analogous maleimide linker.


 Please wait while we load your content...
Please wait while we load your content...