ZnI2/Zn(OTf)2-TsOH: a versatile combined-acid system for catalytic intramolecular hydrofunctionalization and polyene cyclization†
Abstract
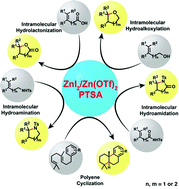
A mild and efficient combined-acid system using a zinc(II) salt [ZnI2 or Zn(OTf)2] and p-toluene sulfonic acid (TsOH) was investigated for catalytic cationic cyclizations, including intramolecular hydrocarboxylation, hydroalkoxylation, hydroamination, hydroamidation, hydroarylation and polyene cyclizations. This reaction provides easy access to five- and six-membered O- and N-containing saturated heterocyclic compounds, tetrahydronaphthalene derivatives and polycyclic skeletons in excellent yield with perfect Markovnikov selectivity and under mild conditions. The operational simplicity, broad applicability, and use of inexpensive commercially available catalysts make this protocol superior to existing methodologies.


 Please wait while we load your content...
Please wait while we load your content...