Promoting proton coupled electron transfer in redox catalysts through molecular design
Abstract
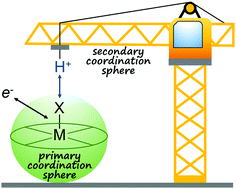
Most bond-forming and -breaking redox reactions require the concomitant transfer of protons. Unassisted proton movement can result in kinetic and thermodynamic barriers that inhibit the rate of these reactions, leading to slow and/or inefficient catalysis. These barriers can be circumvented by effective proton management through molecular design. Different strategies for managing proton movement are discussed with examples from biological and synthetic systems. As proton management is particularly important in redox reactions for chemical fuel generation and utilization, the focus will be on catalysts for H–H and O–O bond formation and cleavage. However, we expect the approaches discussed herein will be general to most multi-electron, multi-proton reactions.
- This article is part of the themed collection: Frontiers in Proton Coupled Electron Transfer (PCET)


 Please wait while we load your content...
Please wait while we load your content...