Unravelling the role of amino acid sequence order in the assembly and function of the amyloid-β core†
Abstract
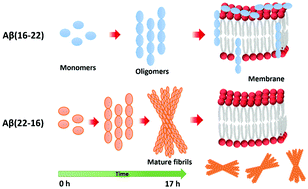
The amino acid sequence plays an essential role in amyloid formation. Here, using the central core recognition module of the Aβ peptide and its reverse sequence, we show that although both peptides assemble into β-sheets, their morphologies, kinetics and cell toxicities display marked differences. In addition, the native peptide, but not the reverse one, shows notable affinity towards bilayer lipid model membranes that modulates the aggregation pathways to stabilize the oligomeric intermediate states and function as the toxic agent responsible for neuronal dysfunction.


 Please wait while we load your content...
Please wait while we load your content...