Ion effects on the conformation and dynamics of repetitive domains of a spider silk protein: implications for solubility and β-sheet formation†
Abstract
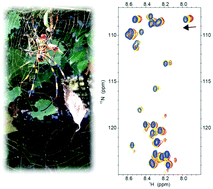
The effect of ions on the structure and dynamics of a spider silk protein is elucidated. Chaotropic ions prevent intra- and inter-molecular interactions on the repetitive domain, which are required to maintain the solubility, while kosmotropic ions promote hydrogen bond interactions in the glycine-rich region, which are a prerequisite for β-sheet formation.


 Please wait while we load your content...
Please wait while we load your content...