An axially chiral 1,1′-biazulene and its π-extended derivative: synthesis, structures and properties†
Abstract
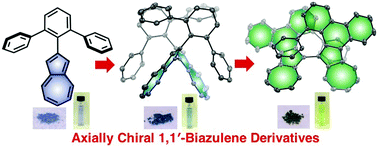
Chiral organic π-conjugated molecules have been intensively investigated in the fields of chiral electronic materials and devices. Herein we report a new motif for an axially chiral 1,1′-biazulene and its π-extended derivative, in which biphenyl was annulated by the electron-rich five-membered-ring of the azulene moiety. These two compounds were unexpectedly synthesized from 2-terphenyl azulene by stepwise intermolecular and intramolecular oxidative C–H/C–H couplings. X-ray diffraction analysis revealed that the crystals of both compounds contain a pair of enantiomers. Furthermore, the axially chiral 1,1′-biazulene derivatives were successfully separated into enantiomers that exhibited clear mirror-image circular dichroism spectra up to the near-infrared region.


 Please wait while we load your content...
Please wait while we load your content...