Acid-catalyzed chirality-transferring intramolecular Friedel–Crafts cyclization of α-hydroxy-α-alkenylsilanes†
Abstract
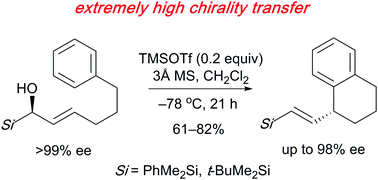
Acid-catalyzed intramolecular Friedel–Crafts cyclization of optically active α-hydroxy-α-alkenylsilanes possessing a benzene ring (>99% ee) with TMSOTf as a Lewis acid gave enantio-enriched tetrahydronaphthalenes (up to 98% ee). The silyl group attached to the chiral carbon played a crucial role in the chirality transfer.


 Please wait while we load your content...
Please wait while we load your content...