Photo electron transfer induced desilylation of N,N-bis(trimethylsilyl)aminodibenzoborole to aminodibenzoborole†
Abstract
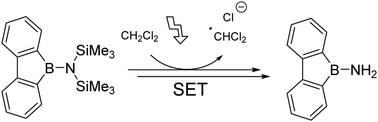
The synthesis of 9-amino-9-borafluorene is described using a photoinduced twofold desilylation of the N,N-bis(trimethylsilyl) derivative 2. The mechanistic analysis suggests an initial single electron transfer step from 2 to the halogen containing solvent. 9-Amino-9-borafluorene undergoes a photoinduced cyclooligomerization, most reasonably to the dimer.


 Please wait while we load your content...
Please wait while we load your content...